K ODK 1 See answer Advertisement Advertisement jordanrbrown04 is waiting for your help. Atomic radius of Oxygen O 152 pm.

Periodic Table Worksheet Answer Key Chemistry Worksheets Periodic Table Atomic Structure
Atomic radius of Nitrogen N 155 pm.

. A Na B Cl C P D Br E K. This is because there occurs increase. Atomic radius of Beryllium Be 153 pm.
Atomic radius of Neon Ne 154 pm. Group by having Lithium and it is Sodium and we know when you move along the period justice decreases due to increase in effective nuclear charge find know if you see it over here that you fluorine over here and nitrogen no doubt your Sodium and Aluminium has the greater size and nitrogen and Fury talking about this to talk about it the more negative charge is present on your. A Se b As c S d Sb e Ge.
Add your answer and earn points. Which of the following are wrong. A Na b Al c N d F.
Which of the following elements have the largest atomic radius. A Li b Cl c Na d None of above. Therefore 1 s 2 2 s 2 2 p 6 3 s 2 has the largest radius.
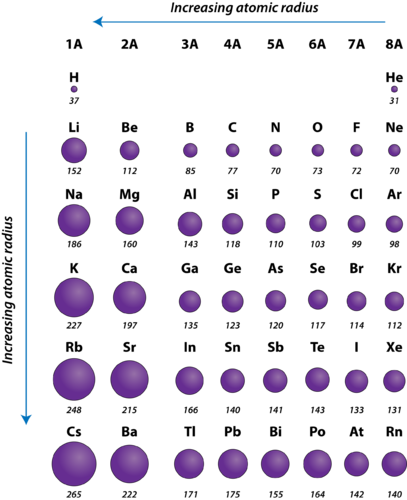
Na and Cl. Use the constants at the end of each sentence to represent the atomic propositions they are obviously meant for. You can confirm the answer by looking at an atomic radius table like this one.
Which one of the following atoms has the largest radius. Thus ionic radius increases. Which of the following has the largest radius.
So the correct answer is A 1 s 2 2 s 2 2 p 6 3 s 2. Which of the following atoms has the largest radius. Atomic radius of Boron B 192 pm.
Which of the following has the bartleby. The effective nuclear charge of an atom is primarily affected by _____. Option c is correct.
Experts are tested by Chegg as specialists in. It is known that when we move down a group then there is increase in size of atom. Na has the largest atomic radius.
Which of the following has the largest radius. 1s 2s 2p 3s 3p3 d 1s2 2s2 2p 3s2 3pS Arrange the. This is because as one electron is removed from the valence shell of the atom the effective nuclear charge on the valence shell decreases.
An atom of which of the following elements has the largest atomic radius. Thus the ionic radius of C s is the largest. A p e x.
Atomic radius of Fluorine F 135 pm. Which of the following has the largest atomic radius. A Rb b Si c S d O.
Which atom has the highest first ionization energy. Which of the following has largest radius 1s2. Atomic radius of Carbon C 170 pm.
Which one of the following atoms has the largest radiusA O B F C S D Cl E Ne. Which one of the following has the smallest radius. Thus O 2 has the largest radius.
P 3 P 3. Chemistry questions and answers. A Be B C C O D F E Ne.
A Cs b Ca c Al d O. Atomic radius of Magnesium Mg 173 pm. Which one of the following atoms has the largest radius.
The element which has the largest atomic radius is Cesium. The anionic radius is difficult to define because an anion has an additional electron which increases the size of the electron cloud and may make. A Na b Cl c Fe d P e Br 3Of the following elements which has the largest first ionization energy.
Which of the following has the largest radius kNaNak Get the answers you need now. ANoble gases have highest IE in respective periods bZnO is amphoteric oxide cIonic radius is inversely proportion asked Sep 24 2019 in Chemistry by AmritKaushik 237k points. Atomic Radius is defined as the distance between the center of the nucleus and the outermost shell of an atom.
Karibegay45 karibegay45 11042020 Chemistry College answered Which of the following has the largest radius kNaNak 2 See answers Advertisement Advertisement gilbabig gilbabig Answer. Thus the correct option is A C s. Which of the following atoms has the smallest radius.
In periodic table 3rd period will have larger atomic radius than the second period. A outer electron b orbital radial probability c nuclear charge d inner electrons e electron distribution. 1Which one of the following atoms has the largest radius.
Cl- Please explain why Expert Answer. A F B S C O D Ne E Cl. Belongs to the 2nd period.
Atomic radius of Sodium Na 227 pm. Cl C l. Which of the following atoms has the largest radius.
S2 S 2. Since potassium is located at the start of period 3 and bromine at the end of the same period potassium will have a larger atomic radius than bromine and thus the largest atomic radius of the four given atoms. Which of the following has the largest radius.
Which of the following has largest radius - a 1s2 2s2 2p 3s b 1s2 2s2 2p 3s2 3p. Which atom has the lowest first ionization energy. A I B Co C Ba.
Who are the experts. While all of these ions and atoms have the 1s22s22p63s23p6 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 electron configuration due to the gain or loss of electrons by ions 3 the sulfide ion S2 has the largest radius. A Sr B Ca C K D Rb E Na.
A Na b Ba c Ca d Cs. Thus helium is the smallest element and francium is the largest. Belong to the 3rd period.
Which of the following has the largest radius. Translate the following English sentences into our symbolic language using any of the three truth functional operators ie conjunction negation and disjunction. Thus atomic radius is directly proportional to the number of electron shells.
A O b F c S d Cl e Ne 2Which one of the following has the smallest radius.

The Parts Of The Periodic Table


0 Comments